Regulation of urea cycle
Downloads: CellDesigner MINERVA
Contributors
Marcio Luis Acencio
Description
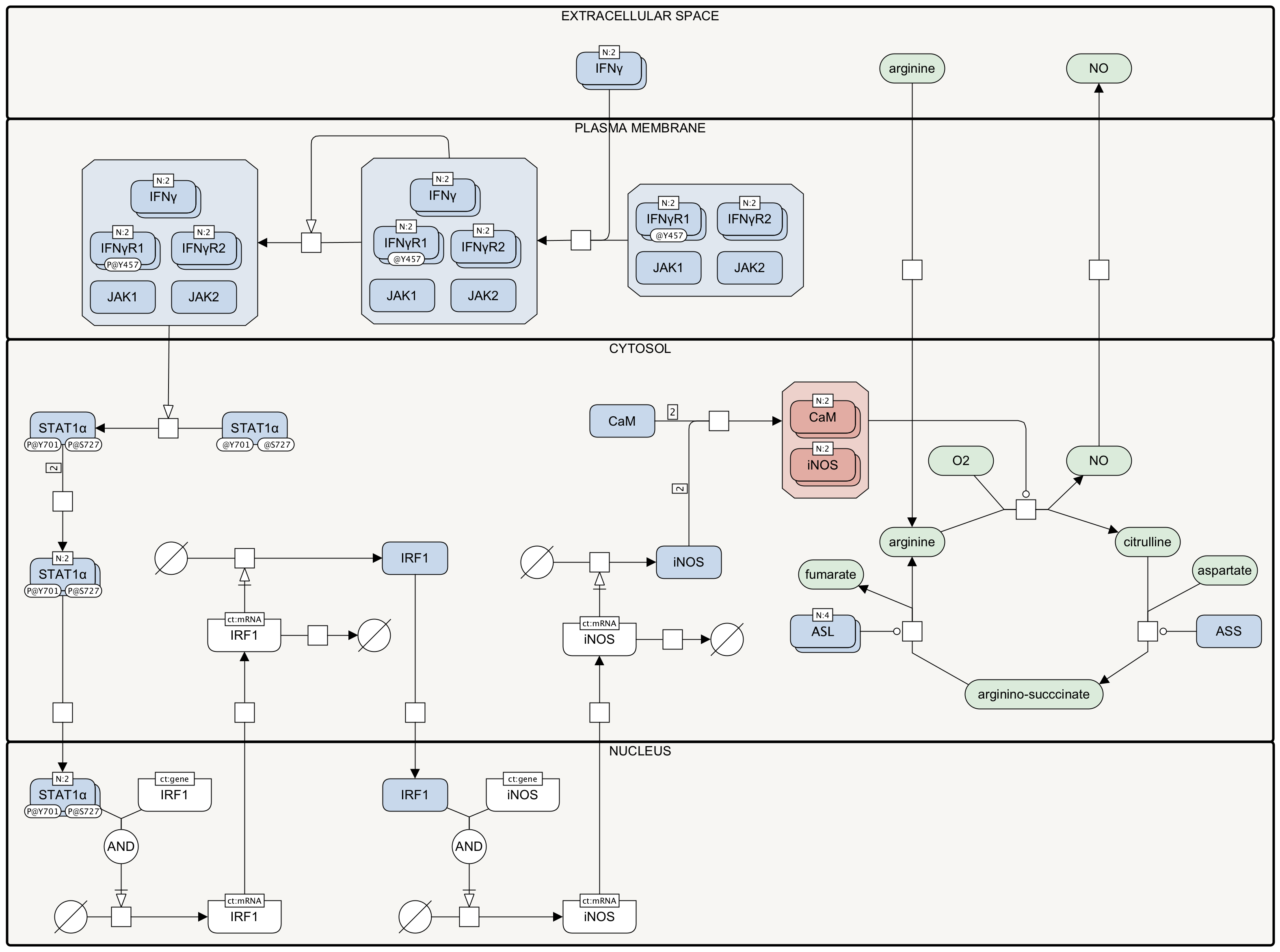
The urea cycle (UC) converts highly toxic ammonia to urea for excretion [1]. It takes place in hepatocytes and consists of five reactions that occur in mitochondria and cytosol. In mitochondria, ammonia (NH3), bicarbonate (HCO3-) and ATP are converted to carbamoyl phosphate, ADP and phosphate (Pi) by the enzyme Carbamoyl-Phosphate Synthase 1 (CPS1). Still in mitochondria, carbamoyl phosphate and ornithine are converted to L-citrulline and Pi by the enzyme ornithine transcarbamylase (OTC) [1]. L-citrulline is then transported to the cytosol through the mitochondrial ornithine-citrulline antiporter SLC25A15, also known as ornithine translocase. This antiporter catalyses the simultaneous exchange between cytosolic ornithine and mitochondrial citrulline [2]. In cytosol, an ATP-dependent condensation reaction catalyzed by the enzyme argininosuccinate synthetase 1 (ASS1) occurs between aspartate and L-citrulline to form argininosuccinate. Argininosuccinate is cleaved by argininosuccinase (ASL) to form L-arginine and fumarate. L-arginine is then cleaved by arginase (ARG1) to form urea and ornithine. While urea is excreted, L-ornithine is transported back to the mitochondria via the antiporter SLC25A15 as described above [1,2].
Besides generating urea, UC is also the main biochemical pathway in which the amino acid arginine is synthesised. Two enzymes are directly linked to arginine biosynthesis, namely, ASS1 and ASL, of which ASS1 is a key regulator not only in hepatocytes UC, but also in the arginine–citrulline cycle that occurs in many extrahepatic tissues [4]. Post-translational modifications (PTMs) can affect ASS1 activity: when acetylated by protein CLOCK, ASS1 is inhibited and when deacetylated by histone deacetylases, ASS1 activity is restored [5]; in addition, as shown in the diagram, ASS1 can be also ubiquitinated and eventually degraded by proteosome in response to low expression of the DNA binding transcription factor snail family transcriptional repressor 1 (SNAI1) and high expression of the long non-coding RNA (lncRNA) LOC113230 [6]. When SNAI1 is present, the expression of LOC113230 is repressed by the binding of SNAI1 to E-box elements located in LOC113230 promoter; otherwise, LOC113230 is expressed and then can recruit LRPPRC‐TRAF2 complex to ubiquitinate ASS1 at its residue K234. Interestingly, it seems that this LOC113230-driven ASS1 ubiquitination and consequent degradation is a tumor suppressor mechanism in which arginine deprivation strongly suppresses growth, migration and metastasis of cancer cells [6].
References
-
Cox, Michael (2013-01-01). Lehninger Principles of Biochemistry. Freeman. ISBN 9781429234146. OCLC 901647690.
- Fiermonte G, Dolce V, David L, Santorelli FM, Dionisi-Vici C, Palmieri F, Walker JE. The mitochondrial ornithine transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution of two human isoforms. J Biol Chem. 2003 Aug 29;278(35):32778-83
- Zhang L, Zou Y, Lu Y, Li Z, Gao F. Unraveling the therapeutic potential of carbamoyl phosphate synthetase 1 (CPS1) in human diseases. Bioorg Chem. 2023 Jan;130:106253.
- Keshet R, Szlosarek P, Carracedo A, Erez A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat Rev Cancer. 2018 Oct;18(10):634-645
- Lin R, Mo Y, Zha H, Qu Z, Xie P, Zhu ZJ, Xu Y, Xiong Y, Guan KL. CLOCK Acetylates ASS1 to Drive Circadian Rhythm of Ureagenesis. Mol Cell. 2017 Oct 5;68(1):198-209.e6
- Jia H, Yang Y, Li M, Chu Y, Song H, Zhang J, Zhang D, Zhang Q, Xu Y, Wang J, Xu H, Zou X, Peng H, Hou Z. Snail enhances arginine synthesis by inhibiting ubiquitination-mediated degradation of ASS1. EMBO Rep. 2021 Aug 4;22(8):e51780